T. Abe1, J.P. Loenneke2, K. Kojima1, R.S. Thiebaud2, C.A. Fahs3, O. Sekiguchi4
1. Department of Kinesiology, School of Public Health, Indiana University, Bloomington, IN; 2. Department of Health and Exercise Science, University of Oklahoma, Norman, OK; 3. Exercise and Sport Science Department, Fitchburg State University, Fitchburg, MA; 4. Nippon Sports Science University, Setagaya, Tokyo, Japan
Corresponding Author: Takashi Abe, PhD, Department of Kinesiology, School of Public Health, Indiana University, 1025 East 7th Street, Room 104, Bloomington, IN 47405, USA. E-mail: t12abe@gmail.com, Phone: +1-(812)-856-7163, FAX: +1-(812)-855-3193
Abstract
Strength training can increase skeletal muscle mass (SM), however, the hypertrophic responses between trunk and limb muscles may differ. This may be problematic because dual-energy X-ray absorptiometry (DXA)-derived appendicular lean mass (aLM) does not include trunk SM. Thus, the purpose was to compare trunk and limb SM (measured by magnetic resonance imaging) between weightlifters (WL) and moderately active men (CON). With the exception of lower-leg SM, WL had greater total and segmental SM than CON. Relative SM, such as trunk to total SM was greater in WL than in CON. Because trunk SM includes the shoulder and hip joints muscles, we reanalyzed major individual muscles of only three subjects (two in CON group and one in WL group). Although WL had greater trunk SM, the DXA-determined aLM does contain these muscles. Thus, these results suggest that the DXA may be used to track SM adaptations to chronic strength training.
Key words: Resistance training, muscle volume, lean tissue mass, MRI.
Introduction
Dual-energy X-ray absorptiometry (DXA)-determined appendicular lean mass (aLM) or bioelectrical impedance analysis (BIA)-estimated total skeletal muscle mass (SM) is a major criteria for diagnosis of age-related loss of SM (1-3). Although a large proportion of SM is observed in the arms and legs including shoulders and glutei (4), DXA-derived aLM does not include trunk SM. A study reported that ~40% of total SM is located in the trunk region of the human body (4). Thus, it is unclear whether aLM and total SM results in a similar criteria for diagnosis of age-related SM loss when SM distribution is different among individuals. A study reported that the prevalence of age-related SM loss varied widely depending on diagnostic criteria and criteria based on total SM failed to match with criteria based on aLM (2).
Strength (resistance) training is recommended to maintain and increase SM in older adults (5). In general, it is thought that the muscle hypertrophic responses are almost identical between trunk and limb muscles.
However, there are only a few studies that have compared muscle hypertrophic responses between trunk and limb muscles following resistance training (6, 7). Unfortunately, those studies measured muscle thickness, not muscle mass, for evaluating the change in muscle distribution by strength training. Thus, it is unknown whether the distribution of segmental muscle mass differs between resistance-trained and non-resistance trained subjects. The purpose of the present study was to compare the trunk and limb SM between weightlifters and moderately active young men.
Methods
Eight male weightlifters (WL) and 8 age- and height- matched moderately active men (CON) were recruited for this study (Table 1). The WL had been training competitively over 5 years and participated in strength training on a regular basis (5 times/week). The strength training programs were high intensity (>80% of one repetition maximum) in nature. The CON had played recreational sports without resistance exercise (1-2 times/week). All subjects received a written description of the study and gave their informed consent to participate prior to testing. This study was approved by the academic institutions Ethics Committee for Human Experiment.
Body density was measured by the hydrostatic weighing technique. Body fat percentage was calculated from body density using an equation (8). Fat-free mass (FFM) was estimated as body mass minus fat mass. The estimated coefficient of variation (CV) of this FFM measurement from test-retest procedures was 0.7%.
Magnetic resonance imaging (MRI) images were prepared using previously described methods (4). Briefly, a T1 weighted, spin echo, axial plane sequence was performed with a 1500 millisecond repetition time and a 17 millisecond echo time. With the first cervical vertebra as the point of origin, contiguous transverse images with 1.0 cm slice thickness (0 cm interslice gap) were obtained from the first cervical vertebra to the ankle joints for each subject (about 150 slices per person). Skeletal muscle volume units (liters) were converted into mass units (kg) by multiplying the volumes by the assumed constant density for SM (1.041 g/ml). The estimated CV of this SM mass measurement from test-retest was 2.1% (4).
Results are expressed as means and standard deviation for all variables. The difference between WL and CON was tested for significance by using unpaired Student’s t- tests. Before comparison groups, data were tested for normality of distribution by the Shapiro–Wilk test and consequently all variables obtained were normally distributed. Pearson product correlations were performed to assess the relationship between total SM and relative segmental SM variables. Significance was set at P ≤ 0.05.
Results
There were no differences in age, height, and percent body fat between the two groups. WL had greater body mass, BMI, and FFM than CON. WL also had greater total and segmental SM than CON, except for lower leg SM. The ratio of appendicular to trunk SM as well as trunk to total SM was significantly difference between the groups (Table 1). Trunk to total SM ratio was positively correlated to total SM (r = 0.504, p = 0.046) when the overall sample was used.
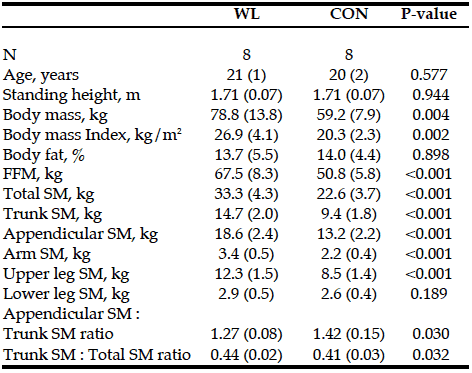
Table 1: Total and segmental skeletal muscle mass and body composition in resistance trained (WL) and nonresistance trained moderately active men (CON)
SM, skeletal muscle mass; FFM, fat-free mass; Appendicular = arm + upper leg + lower leg
Discussion
Our finding showed that WL had higher proportional trunk SM compared to CON, and the ratio of trunk to total SM was associated with total body muscularity. In general, training programs are composed of multiple- joint trunk and limb exercises as well as single-joint limb exercises. Thus, training volumes may be greater in the limbs than in the trunk muscles, which may affect the segmental SM distribution. Following a single mode of bench-press training, however, training-induced increase in muscle thickness was greater in the trunk muscle compared to the limb muscle (7). Because trunk SM measured in this study includes the shoulder and hip joint muscles, we reanalyzed major individual muscles in the upper- and lower-body of the lowest and the highest total SM in the CON group and the highest total SM in the WL group. As a result, greater muscle hypertrophic adaptations were located in the shoulder (e.g., deltoid SM was 0.47 and 0.81 kg for the CON and 1.25 kg for the WL) as well as the hip (e.g., gluteus maximus SM was 1.22 and 1.66 kg for the CON and 3.35 kg for the WL) joint muscles in the WL compared to the CON. DXA-derived aLM does not include trunk SM, however, the SM in the shoulder and hip joint muscles are included into the DXA- determined aLM. Therefore, even if strength training induced greater SM in the shoulder and hip joints, DXA- derived aLM may contain these changes in SM, except for the iliopsoas muscle.
In conclusion, with the exception of lower-leg SM mass, WL had
greater total and segmental SM than CON. Trunk to total SM ratio was also greater in WL than in CON. Thus, weightlifting induced increases in SM are not proportional in each muscle and the trunk has a greater increase relative to the limb segments. Fortunately, greater muscle hypertrophic adaptations were located in the shoulder and hip joint muscles. Therefore, while DXA-derived aLM does not include whole trunk SM, the SM in the shoulder and hip joint muscles are included into the DXA estimate of aLM. Thus, DXA-determined aLM may contain these greater muscle adaptations even if muscle hypertrophy is not proportional.
Conflict of interest statement: None of the authors had financial or personal conflict of interest with regard to this study.
References
1. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross R, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147:755–763.
2. Bijlsma AY, Meskers CGM, Ling CH, Narici M, Kurrle SE, Cameron ID, Westendorp RG, Maler AB. Defining sarcopenia: the impact of different diagnostic criteria on the prevalence of sarcopenia in a large middle aged cohort. Age (Dordr) 2013;35:871–881.
3. Janssen I, Heymsfield SB, Baumgartner RN, Ross R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol 2000;89:465–471.
4. Abe T, Kearns CF, Fukunaga T. Sex differences in whole body skeletal muscle mass measured by magnetic resonance imaging and its distribution in young Japanese adults. Br J Sports Med 2003;37:436–440.
5. American College of Sports Medicine. Quantity and quality of exercise for developing and maintaining cardiorespitatory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med Sci Sports Exerc 2011;43:1334–1359.
6. Abe T, DeHoyos DV, Pollock ML, Garzarella L. Time course for strength and muscle thickness changes following upper and lower body resistance training in men and women. Eur J Appl Physiol 2000; 81: 174–180.
7. Ogasawara R, Thiebaud RS, Loenneke JP, Loftin M, Abe T. Time course for arm and chest muscle thickness changes following bench press training. Interv Med Appl Sci 2012;4:217–220.
8. Brozek J, Grande F, Anderson JT, Keys A. Densitometric analysis of body composition: revision of some quantitative assumptions. Ann NY Acad Sci 1963;110:113–140.
