E. Miyamoto1,2, M. Kaneko3, S. Ichimaru4, Y. Hokotachi5, T. Amagai6
1. Department of Nutrition, Sonoda Women’s University; 2. Administration Food Science and Nutrition Major, Graduate School of Human Environmental Science, Mukogawa Women’s University; 3. Department of Respiratory Medicine, Kobe City Hospital Organization Kobe City Medical Center West Hospital; 4. Department of Nutrition, Osaka Saiseikai Nakatsu Hospital; 5. Department of Clinical Nutrition, Takarazuka Dai-ichi Hospital; 6. Department of Food Sciences and Nutrition, School of Human Environmental Sciences, Mukogawa Women’s University
Corresponding Author: Teruyoshi Amagai, Mukogawa Women’s University, Nishinomiya, 633-8558, Japan, amagaipedteruyoshi@gmail.com
J Aging Res Clin Practice 2016;5(3):147-154
Published online June 23, 2016, http://dx.doi.org/10.14283/jarcp.2016.103
Abstract
Abstract: Background: In a survey of 2004 conducted by World Health Organization, chronic obstructive pulmonary disease (COPD) is proved third leading cause of death worldwide and develops frailty. However, the differences between pre-frailty and non-frailty or frailty are not well studied. Objective: To examine the working hypothesis that there is differences in phenotypes between pre-frailty and non-frailty or frailty in patients with COPD. Design and setting: This study is a cross-sectional study. Participants: Patients with consecutive COPD male patients, whose age is 50 year-old or older, visited COPD outpatient clinic at single institute, between 2011, March and August, were eligible. Measurements: The data categorized into seven domains according to Fried’s phenotypic criteria were collected in all subjects divided into three groups: non-, pre-, and frailty. All data was compared between two of three groups. Results: Pre-frail COPD patients showed declines in upper extremities’ muscle volume and functions assessed by arm muscle area and hand-grip strength (P<0.05) compared with data in non-frailty group. This was different from results of comparison data between pre- and frailty group where lower extremities’ muscle data showed statistically different (p<0.05). Conclusion: Our observation could be interpreted that declines in upper extremities’ muscle volume and function might precede in comparison pre-frailty with non-frailty COPD patients. This might be an early predictor to progressing the severity of COPD in pre-frail COPD patients and might be potential factor to prevent deterioration of frailty and COPD severity.
Key words: COPD, pre-frailty, upper arm, muscle volume, muscle function.
Introduction
Chronic obstructive pulmonary disease (COPD), which most commonly results from the long-term inhalation of toxic substances produced by smoking, is primarily defined as a disease of airflow obstruction. A 2004 survey conducted by the World Health Organization (WHO) (1) reported that patients with COPD frequently experience systemic inflammation and secondary consequent malnutrition. An additional primary consequence of this condition is a decrease in muscle function. Patients with COPD also develop frailty, conceived as the impairment of physical, psycho-mental, or social function resulting in the decline in physical capabilities and quality of life. Freid (2) defined phenotypic frailty as follows: 1) weight loss, 2) subjective fatigue, 3) reduction in activities of daily life (ADL), 4) attenuation of physical capability, and 5) impairment of hand-grip strength. COPD patients who meet none of these five items are considered non-frail, while those meeting one or two are diagnosed with pre-frailty, and those meeting three or more with frailty. Although several studies have reported that frail COPD patients show poorer outcomes than non-frail patients, little is known about the clinical differences between pre-frail and frail or non-frail COPD patients. If differences were identified in these pre-frail patients, they might be used to predict the development of pre-frailty from non-frailty and frailty from pre-frailty. Moreover, such predictors might also be utilized as the basic platform of preventive strategies. To our knowledge, however, no predictors of the deterioration of non-frailty to frailty in COPD have yet been identified.
To assure this, we hypothesized that differences in findings between non- and pre-frailty or between pre-frailty and frailty in patients with COPD might predict the progression of pre-frailty as the middle status between non-frailty and frailty. Here, to examine this hypothesis, we compared a range of variables in male COPD patients visiting the COPD outpatient clinic at a single institution.
Methods
Subjects
Subjects were consecutive COPD male patients aged 50 years or older who visited the COPD outpatient clinic at our institution between March and August, 2011, and completed the written informed consent. Subjects who missed one or more data collections were excluded from this study.
Collected data
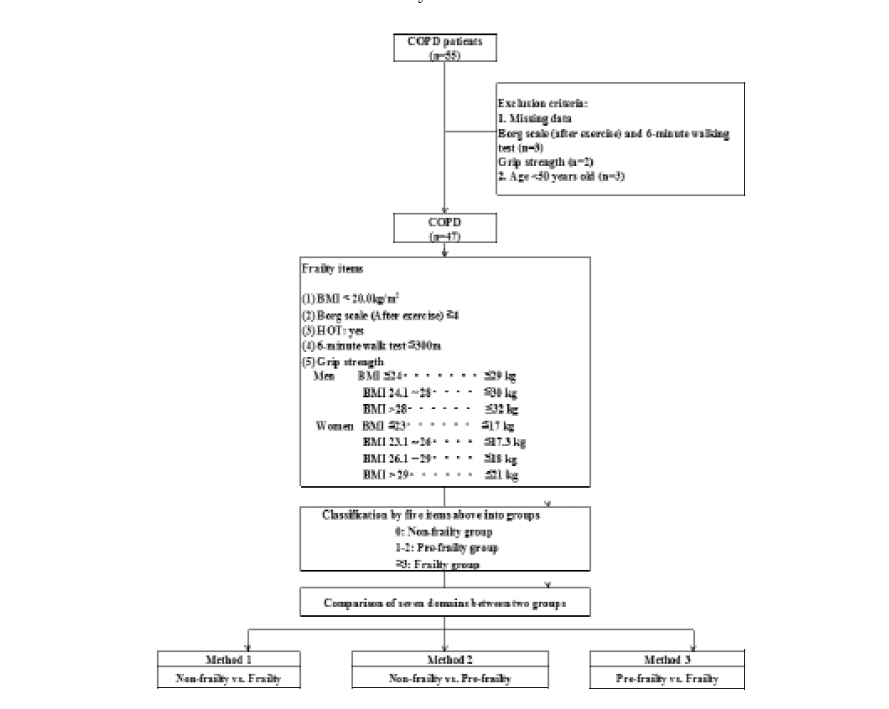
Data were collected in the three different time categories of before, at, and after visit at COPD outpatient clinic in single institute (Fig.1). The collected data were classified in the following seven domains: (1) Demographic domain included age, sex, smoking status, and Brinkman Index (BI) defined as the number of cigarettes smoked per day multiplied by the number of years of smoking. (2) Physical domain included arterial partial pressure of oxygen (PaO2) and carbon dioxide (PaCO2), percutaneous PaO2 (SpO2) flowing in the papillary artery with warming, physical measurement of grip strength, six-minute walking test (6 MWT) (3) and gait speed expressed in meter/second (m/sec) (4), Borg scale and average pulse rate (beat per minute, bpm) during and ten minutes after 6 MWT, and pulmonary capacity measured by spirometry, including forced expiratory vital capacity of one second (FEV1) and %FEV1 (FEV1 divided by forced vital capacity, FVC). In this physical domain, hand grip strength was measured in the non-dominant arm in duplicate, and average value was calculated to express the z-score as a measure of variation from the age- and sex-specific mean value using physical and exercise capacities published in 2014. 6 MWT was defined as maximal walking distance using a standardized 50 meter circular lane, and sense of exhaustion at completion to evaluate subjective exhaustion included in Freid’s frailty category was subjectively evaluated using the Borg scale. (3) Blood test domain included complete blood counts and serum biochemistries (details of biochemistry in Fig.2). (4) Anthropometric domain included anthropometric measurements of height, weight, body mass index (BMI; calculated by weight in kilograms divided by squared height in meters, kg/meter2), mid-upper arm circumference (AC), triceps skin-fold (TSF), calf circumference (CC; here defined as arm muscle circumference calculated by AC divided by AC2 divided by 4*π), and arm muscle area (AMA; calculated by (AC–TSF *π)2/12*π). Here, calculated AMC and AMA values by AC and TSF above mentioned were standardized as % AMC and % AMA, calculated by dividing by the age- and sex-specific mean values collected by JARD 2001 which was published for measured race-specific data among all age subjects in both sexes proved as standard. Height was set to the nearest 0.1 cm as measured with a stadiometer and weight to the nearest 0.1 kilogram as measured with a digital weighing scale. All measurements for AC, TSF for the dominant arm, and CC for the contralateral leg were measured in duplicate and expressed as the average value. The mini-nutritional assessment short-form (MNA-SF) (5) survey was also conducted. (5) Comorbidity domain included the frequency scale for the symptoms of gastro-esophageal regurgitation disease (FSSG) (6), hospital anxiety and depression scale (HAD) (7), and self-rating questionnaire for depression (SRQ-D) (8). All of these were originally developed for COPD patients. (6) Chronic obstructive pulmonary disease (COPD) domain included clinical stage of COPD severity categorized by international guidelines (Global Initiative for COPD: GOLD), dependences of home oxygen therapy (HOT) and of non-invasive intermittent positive ventilation (NIPPV), presence of chronic respiratory failure defined as stage IV diagnosed by GOLD, self-administered COPD assessment test (CAT) (9), and the medical research council dyspnea scale (MRC) (10) for assessing the self-rated severity of COPD. Here, the staging system of GOLD was ranked by %FEV1 defined as measured FEV1 divided by predicted FEV1 calculated as age-, sex-, and height-function, stages of COPD are as the follows: FEV1 ≥ 80% for stage I, ≥50% to <80% for stage II, ≥30% to <50% for stage III, and <30% for stage IV. (7) Nutrient intake domain included daily nutrient intake measured by a self-administrated food frequency questionnaire (brief dietary history questionnaire: BDHQ) assured as well-validated methodology for both healthy and ill subjects (11) , and all obtained data were expressed in units per 1,000 kilocalorie (kcal) as calculated densitometry.
Each timing to collect all data defined in each domain, categorized as before, at, and after outpatient clinic visit, is shown in Fig.2.
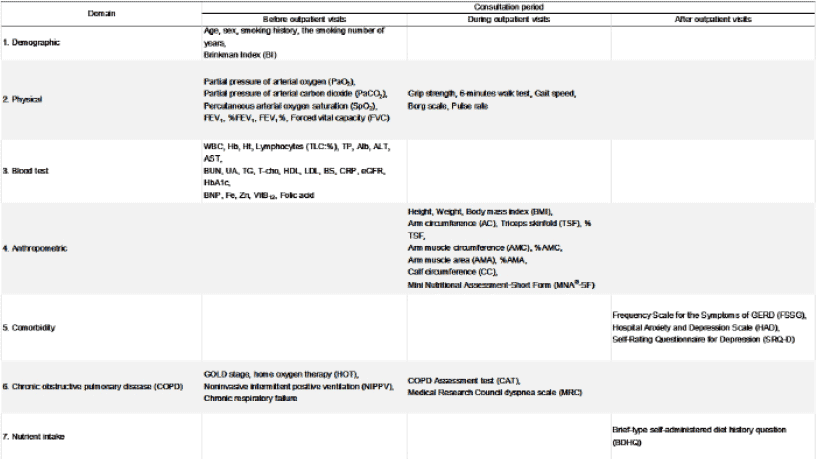
Figure 2
Data collecting and timing of collection before, on, and after visiting the chronic obstructive pulmonary disease (COPD) clinic
Definition of Frailty, Pre-frailty and Non-frailty
Frailty, pre-frailty and non-frailty were defined according to Freid’s criteria for phenotype frailty (2) as follows: (1) BMI < 20.0 kg/m2 for weight loss(12), although BMI is not dynamic but static status, (2) Borg scale ≤ 4 after 6 MWT as an evaluation of subjective exhaustion, (3) dependence on HOT in evaluation of physical activity in daily life, (4) 6 MWT distance ≤ 300 meters for walking distance, (5) grip strength set at the sex- and BMI-specific criteria of 29, 30, and 32 kg for BMI ≤ 24.0, 24.1–28.0, and > 28 kg/m2 for males and 17, 17.3, 18.0, and 21 kg for BMI ≤23.0, 23.1–26.0, 26.1–29.0, and ≥29 kg/m2 for females, respectively (Figure 1).
All subjects were categorized into these three groups, and all data in the seven domains were compared among the three groups in two-group pairs, namely non-frail and frail patients (Method 1), non-frail and pre-frail patients (Method 2), and pre-frail and frail patients (Method 3) shown in Table 1, 2 and 3, respectively. The comparisons of pre-frail COPD patients vs. non- and frailty groups has not been reported anywhere by the present study report.
Statistical analysis
We showed the statistic that we found in each item with the median (25% ile, 75% ile), and the significant difference of each group performed Kruskal-Wallis test, and Steel-Dwass examined a significant difference by Kruskal-Wallis test for an investigation item.
In all tests, p <0.05 was identified a statistically significant difference.
We held Kruskal-Wallis authorization in Package PASW 20.0 for windows (SPSS Inc,Chicago,IL), and the statistical analysis was given Steel-Dwass test in Excel statistics 2010 (Social Survey Research Information Co.Ltd.,Tokyo).
Results
Comparison of Older Non-frail and Frail COPD Patients –Result of Method 1
Significant differences between non-frail and frail COPD patients were seen for all data except sex, smoking status, Brinkman index, and FSSG, HAD and SRQ-D as comorbidity indexes (Fig.1). Frail patients showed greater progression of COPD severity diagnosed by GOLD staging system than the non-frail group, as well as in CAT and MRC scores. Moreover, the frailty group also showed loss of hand grip strength, and decreases in walking speed, hemoglobin concentration, and muscle volume as assessed by AC, AMC, AMA, and CC (Table 1). Frail COPD patients seemed to be at a more advanced COPD stage and to have lost muscle volume and physical function, as shown by hand grip strength, 6 MWT, and gait speed, although it remains unclear whether the relationship between the progression of COPD severity and declines in muscle volume and function in the upper and lower extremities are causality association.
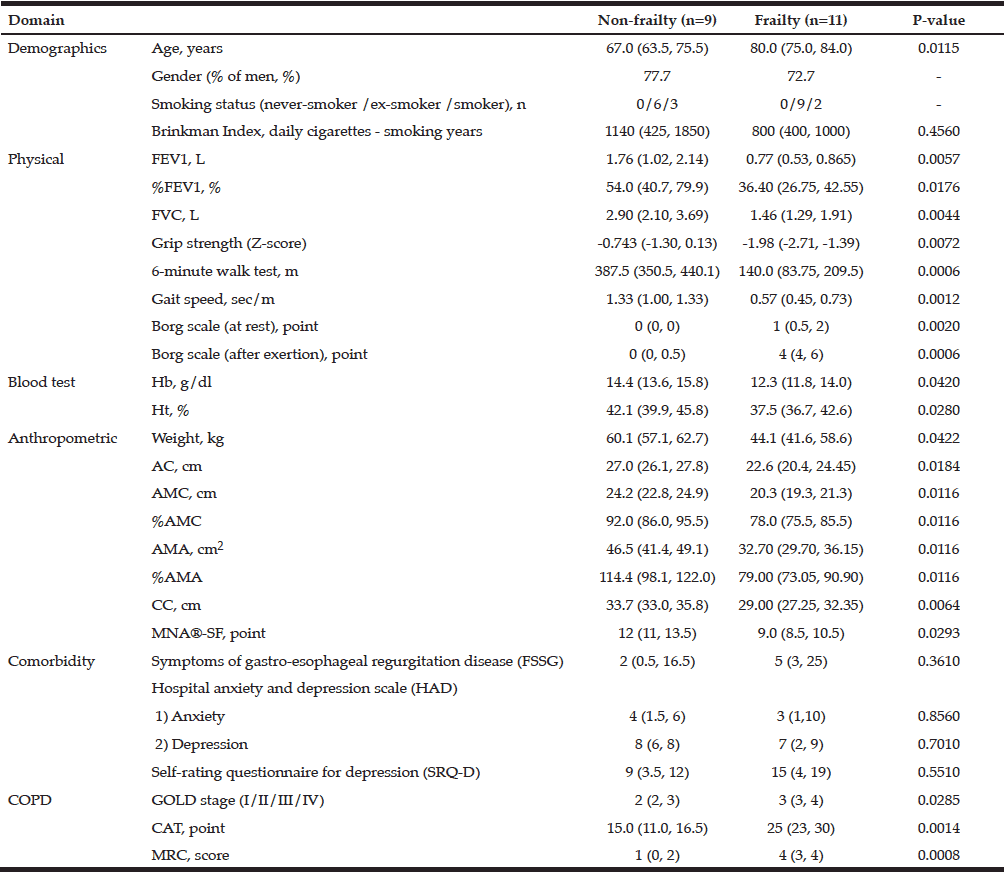
Table 1
Characteristics of non-frailty and frailty patients with chronic obstructive pulmonary disease (COPD)
All data except gender (% men) and smoking status are expressed as median (25th, 75th percentile).
By Freid’s frailty category, subjects with no items were identified non-frailty, 1 or 2 item corresponding pre-frailty, 3 or more items was the frailty.
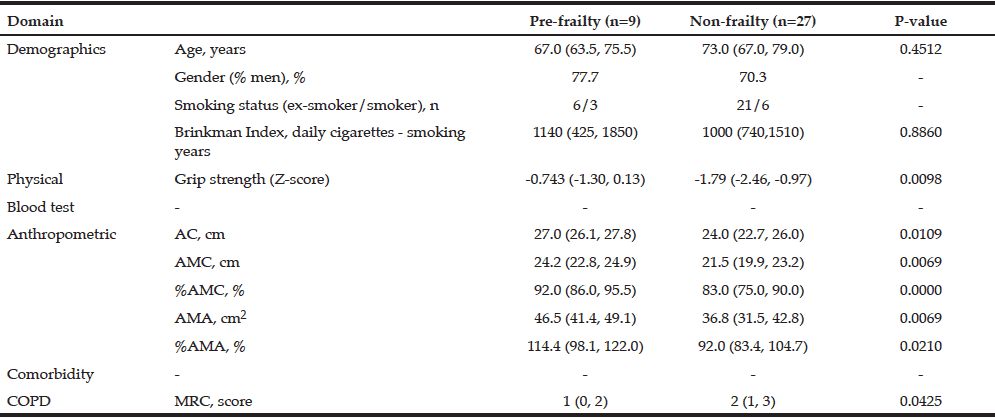
Comparison of Older Non-frail and Pre-frail COPD Patients –Result of Method 2
Excluding demographics data, pre-frailty patients had significantly lower values for AC, AMC, %AMC, and z-score of grip strength than non-frail patients (all, p < 0.05, Table 2). In contrast, other physical and COPD parameters did not show significant differences. This pattern differs from that seen on comparison of the frail and non-frail groups in Method 1 (Fig.1). Accordingly, these variables showing differences in the pre-frailty group might be utilized as early predictors of advancing frailty in COPD patients.
All data except gender (% men) and smoking status are expressed as median (25th, 75th percentile); By Freid’s frailty category, subjects with no items were identified non-frailty, 1 or 2 item corresponding pre-frailty, 3 or more items was the frailty.
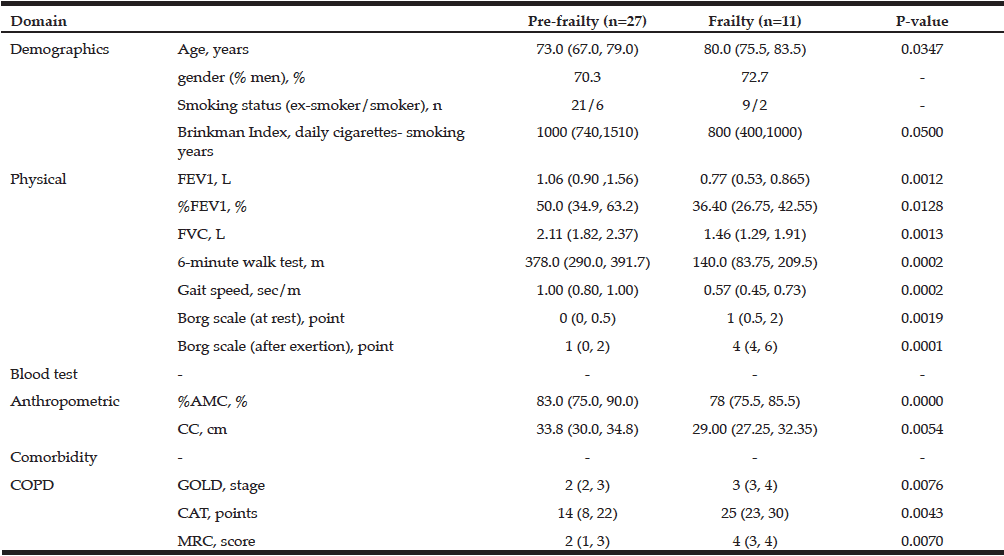
Comparison of Older Pre-frail and Frail COPD Patients –Result of Method 3
Significant differences between pre-frail and frail patients were seen in age, FEV1, %FEV, FVC, %AMC, CC, gait speed, and Borg scale at rest and after exercise as physical parameters, and in GOLD stage and CAT as subjective scores of COPD severity (Fig.2). These differences were completely different to those between the non- and pre-frailty groups, which showed decreased hand grip strength in the pre-frailty group (Table 3). The frailty group showed significant progress in three domains, namely the physical, anthropometric, and COPD domains. Unlike with the non-frail vs. frail comparison in Method 1, statistical differences in lower extremity muscle functions, including 6 MWT and gait speed, appeared to arise later in the development of frailty.
All data except gender (% men) and smoking status are expressed as median (25th, 75th percentile); By Freid’s frailty category, subjects with no items were identified non-frailty, 1 or 2 item corresponding pre-frailty, 3 or more items was the frailty.
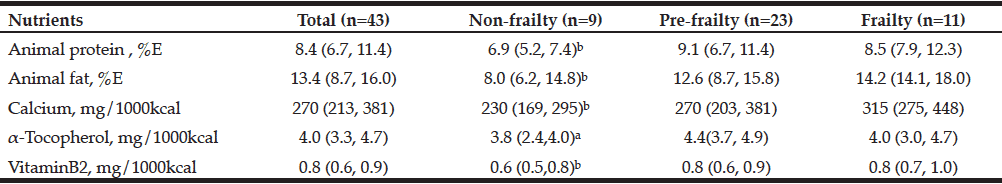
Comparison of Nutrient Intake among the Non-, Pre-, and Frailty Groups
Additionally nutrient intake assessed by dietary questionnaire named BDHQ among three groups diagnosed by Freid’s frail category were compared to examine the differences nutritionally intakes among them. As the results of this comparison, intake of alpha-tocopherol intake was higher in the pre-frailty than in non-frailty group (p < 0.05, Table 4). No difference was seen in the intake of other nutrients. Nutrient intake of four nutrients – animal protein, animal fat, calcium, and vitamin B2 was higher in the frailty than in the non-frailty group. These results were surprisingly opposite to our speculations that inverse relation where frail subjects might take less than the other groups as generally acceptable. One reason to explain the observed nutrient intake results could be that intake was analyzed with a food frequency questionnaire (FFQ), namely the brief-type self-administered diet history questionnaire (BDHQ), in which the results are corrected for energy density. Frail patients might have less energy amount, and our results for pre-frailty and frail patients might accordingly have been overestimated, although any dietary assessing methods, including BDHQ, have acceptable range to assess accurately daily energy intake mentioned in governmental overview report published in 2015 (13).
All data was expressed in median (25th, 75th percentile); a: vs. Pre-Frailty, b: vs. Frailty, p<0.05; By Freid’s frailty category, subjects with no items were identified non-frailty, 1 or 2 item corresponding pre-frailty, 3 or more items was the frailty; This was Kruskal-Wallis test for each BDHQ result in Frailty group-specific. Significant differences among items between groups were compared with the Steel-Dwass test.
Discussion
Frailty in Patients with COPD
Frailty has been shown to be independently associated with several clinical morbidities and treatments, including myocardial infarction(14), cardiac surgery(15), cardiac interventions(16), femoral neck fracture(17), hemodialysis(18), and critical illness(19). In these studies, older age and frailty were associated with poor outcomes. Although the prevalence of COPD is increasing worldwide, the association of frailty with outcomes in patients with COPD has not been widely investigated. The present study enrolled older adults with COPD visiting a COPD clinic during a six-month period. the prevalence of frailty, non-frailty including both of non-frailty and pre-frailty, when pre-frailty could not identified in the most studies, were 22% (11 out of 49) and 78% (38 out of 49), respectively in our subjects, whereas the prevalence of frailty are reported to vary from 57.8% (20) in the community-dwelling population to 46.5% in hospitalized patients (21). Focusing on the deteriorating changes from non-frailty to pre-frailty and from pre-frailty to frailty in patients with COPD might provide clues to identifying populations at high risk of developing frailty or pre-frailty, and analysis of data from patients categorized by Freid’s frailty phenotype might aid to develop strategies for prevention and treatment of frail older patients with COPD. This explains in part why we analyzed data from patients visiting our COPD clinic classified into the three severity categories of non-, pre-, and frailty.
Potential Predictors of Progression of Non-, Pre- and Frailty in COPD patients
(1) Decrease in Upper Extremity Muscle Function and Volume as Potential Predictor of Progression from Non-frailty to Pre-frailty
To test our working hypothesis that there are predisposing factors to predict the progression of frailty phenotype in COPD patients from non-frailty to pre-frailty or from pre-frailty to frailty, we compared all collected data between non- and pre-frailty COPD older patients in method 2. Results showed that upper extremity muscle function assessed by hand grip strength, and muscle volume calculated by arm muscle circumference (AMC), upper arm muscle area (AMA) and %AMA, were all significantly smaller in COPD patients with pre-frailty than in those with non-frailty. These variables might be utilized as predictors of the progression of frailty stage from non-frailty to pre-frailty. To our knowledge, this is first report that a decline in upper extremity muscle volume and function might precede impairments of the lower limb muscle volume and function assessed by gait speed and calf circumference. Although gait speed and calf circumference are known to be sensitive indicators of malnutrition in older adults, our observation might indicate that decreases in the volume and function of upper limb muscles might decline earlier than those in the lower limb. This observed phenomenon might result from hyperactivity of the thorax frequently observed in patients with COPD, such as tachypnea and forced respiratory movements. However, the mechanism underlying this observation has not been fully explained, and further studies with a larger number of subjects are warranted.
(2) Decrease in Lung Function and Performance as Potential Predictor of deterioration from Pre-frailty to Frailty
Contrary to our expectation that the above observational results for potential predictors of deterioration from non-frail to pre-frail COPD patients might be consistent, comparison of data between the pre-frailty and frailty groups showed that parameters of COPD domain (Fig.2), including FEV1, FVC, GOLD stage, and CAT, showed greater decline in the frailty than the pre-frailty group (Table 3). These results might be interpreted that the progression from pre-frailty to frailty might be detected by deterioration in COPD severity, and not by the change in upper limb muscle parameters observed in result 1. However, calf circumference as an index of lower extremity muscle volume was significantly smaller in the frail than the pre-frail group (33.8 vs. 29.0 mm, respectively; p=0.000). This finding might also indicate that muscle changes in the upper extremities precede that in the lower extremities, as mentioned above.
Three-quarters of our COPD subjects were categorized as non-frail or pre-frail. Average diagnosis in these two groups according to the GOLD criteria was stage II (Tables 2). The overall severity of frailty in COPD patients treated at the outpatient clinic appears related to the severity of COPD graded by the GOLD staging system. Although it remains unclear whether the relationship between the progression of frailty and severity of COPD is causality association, prevention or slowing the progression of COPD severity from early to advanced stages by preventing the progression of frailty itself might be reasonable. In other words, the earlier identification of deterioration from non-frailty to pre-frailty might supportive to prevent progression in the severity of COPD. If so, early detection of declines in arm muscle strength and mass might help protect against the disease and frailty progression.
Comparison of daily nutrient intake among the three groups by BDHQ
To validity and clinical feasibility of evaluating daily nutrient intake using the BDHQ has been confirmed. Five nutrients evaluated with the BDHQ showed significant differences between any two groups categorized by frailty. Daily intake of alpha-tocopherol was significantly larger in the pre-frailty than the non-frailty group. Daily intakes of four other nutrients appeared significantly larger in the frailty than the non-frailty group. Contrary to our expectation, these results might suggest that the pre-frailty and frailty groups tend to have higher nutrients’ density in unit energy of 1,000 kcal. Although it is reported that daily energy intake cannot be measured by any methodology, the nutrient intake in Table 4 might not reflect the daily intake of individual nutrients. In other words, our BDHQ findings suggest that the pattern of nutrient intake of pre-frail or frail COPD patients might likely differ from that of non-frail patients. A conclusive understanding of nutrient intake in pre- and frail COPD patients awaits further analysis. The results were contrary to our expectation. This inverse relationship have also been reported elsewhere (22). One reason might be that daily nutrient intake analyzed by food frequency questionnaire (FFQ), including the BDHQ used in the present study, is shown as energy-density corrected, whereas energy consumption by frail patients might be relatively low. This would have tended to overestimate intake for pre- and frail patients.
Study limitations
Several limitations of our study warrant mention. First, the number of subjects was too small to draw definitive conclusions. Second, the study was conducted under a cross-sectional retrospective design, and further evidence from a large prospective study is required. Third, the mechanism to explain the observed phenomenon is that muscle changes in the arms precede those in the legs in frail COPD patients. The question of whether this phenomenon is consistent in the early phase of frailty and its association with COPD severity warrants further investigation. Finally, our study does not indicate whether the early detection of progression in COPD and frailty stage by upper muscle changes will contribute to the treatment of these conditions. Answering these questions requires further intervention studies using nutritional supplementation, including energy and protein.
Conclusion
Our study suggests that muscle volume in the arm and function as measured by hand grip strength might predict earlier change in pre-frail patients with COPD compared with non-frail patients treated on outpatient basis. Furthermore, frail patients might have a significant decline in lung function, COPD progression as measured by GOLD stage, and reduced lower extremity function as measured by the 6-minute walking test, gait speed, and calf circumference compared with pre-frailty patients. Changes in upper extremity muscle volume and function might be able to be as predictors to identify COPD patients in the early phase of pre-frailty from non-frailty. These changes appear to be followed by a decline in lung function and lower extremity muscle changes as observed in frail patients. These findings suggest that categorization of frailty using pre-frailty might be a useful way to early identifying change in COPD outpatients.
Conflict of Interest: The all authors have no conflict of interest to disclose.
Author’s contribution: Eri Miyamoto conducted date analyses and drafted the manuscript. Teruyoshi Amagai, Masahiro Kaneko and Satomi Ichimaru contributed to the conception and design of the study. Teruyoshi Amagai and Satomi Ichimaru participated in the interpretation and discussion of the data and made critical revisions to the manuscript. All authors read and approved the final manuscript.
Ethics Statement: This study was conducted according to the guidelines in the Declaration of Helsinki, and procedures were approved by the Ethics Committee of Kobe City Medical Center West Hospital.
References
1. THE GLOBAL BURDEN OF DISEASE, http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_full.pdf?ua=1, Accessed date, October 27, 2015), Deaths COPD has been reported to be the fourth largest.
2. Freid LP, Tangen CM, Walston J et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Bio Soc Med Soc 2001;56: M146-M156.
3. Masahiro Satake, Takanobu Shioya, Sachiko Uemura et al. Dynamic hyperinflation and dyspnea during the 6-minute walk test in stable chronic obstructive pulmonary disease patients Int J Chron Obstruct Pulmon Dis. 2015;10: 153–158.
4. Samantha S.C. Kon, Jane L. Canavan, Claire M. Nolan et al. The 4-metre gait speed in COPD: responsiveness and minimal clinically important difference. Eur Respir J. 2014;43(5):1298-305
5. Yoshikawa M, Fujita Y, Yamamoto Y et al. Mini Nutritional Assessment Short-Form predicts exacerbation frequency in patients with chronic obstructive pulmonary disease. Respirology;2014;19(8):1198-203
6. Yasuo Shimizu, Kunio Dobashi, Motoyasu Kusano et al. Different gastoroesophageal reflux symptoms of middle-aged to elderly asthma and chronic obstructive pulmonary disease (COPD) patients. J Clin Biochem Nutr; 2012;50(2): 169–175
7. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. Jun; 1983;67(6):361-70
8. Rockliff BW. A brief self-rating questionnaire for depression (SRQ-D). Psychosomatics; 1969;10(4):236-43.
9. Jones PW, Harding G, Berry P et al. Development and first validation of the COPD Assessment Test. Eur Respir J 2009;Sep;34(3):648-54
10. Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23(6):932-46
11. Kobayashi S, Honda S, Murakami K et al. Both comprehensive and brief self-administered diet history questionnaires satisfactorily rank nutrient intakes in Japanese adults. J Epidemiol. 2012;22(2):151-9.
12. Buttery AK, Busch MA, Gaertner B et al. Prevalence and correlates of frailty among older adults: findings from the German health interview and examination survey. BMC Geriatr 2015;15:22. doi: 10.1186/s12877-015-0022-3. http://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/Overview.pdf. Accessed 10, March, 2016.
13. Ministry of Health, Welfare, and Labors, Japanese government: Overview of dietary reference intake for Japanese (2015).
14. Ekerstad N, Swahn E, Janzon M et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation 2011;124:2397-2404.
15. Sundermann SH, Dedemasch A, Seifert B et al. Frailty is a predictor of short- and mid-term mortality after elective cardiac surgery independently of age. Interact CardioVasc Thorac Surg 2014;18:580-5. doi: 10.1093/icvts/ivu006.
16. Rowe R, Iqbal J, Murali-krishnan R, et al. Role of frailty assessment in patients undergoing cardiac interventions. Open Heart 1:e000033. doi: 10.1036/openhrt-2013-000033.
17. Patel KV, Brenman KL, Brennan ML, et al. Association of a modified frailty index with mortality after femoral neck fracture in patients aged 60 years and older. Clin Orthop Relat Res 2014;472:1010-1017. doi: 10.1007/sl1999-013-3334-7.
18. Salter ML, Gupta N, Massie AB, et al. Perceived frailty and measured frailty among adults undergoing hemodialysis: a cross-sectional analysis. BMC Geriatir 2015 doi: 10.1186/s12877-015-0051-y.
19. Bagshaw S, Stelfox T, McDermid RC et al. Association between frailty and short- and long-term outcomes among critically ill patients: a multicenter prospective cohort study. CMAJ 2014;186:e95-e102. doi: 10.1503/cmaj.130639.
20. Park SK, Ricardson CR, Holleman RG, et al. Frailty in people with COPD, using National Health and Nutritional Evaluation Survey dataset (2003-2006). Heart Lung 2013;42:163-170. doi: 10.1016/j.hrtlng.2012.07.004.
21. Oliveira DR, Bettinelli LA, Pasqualotti A et al. Prevalence of frailty syndrome in old people in a hospital institution. Rev Latino-Am. Enfermagem 2013;21:891-8.
22. Kobayashi S, Asakura K, Suga H et al. High protein intake is associated with low prevalence of frailty among old Japanese women: a multicenter cross-sectional study. Nutr J 2013;12: 164.