K. Bojanowski1, R. Bojanowski1,2
1. Sunny BioDiscovery, Inc. Santa Paula, CA, USA; 2. Scientist emeritus, Polish Academy of Sciences, IOP, Sopot, Poland
Corresponding Author: Krzysztof Bojanowski, Sunny BioDiscovery, Inc. 972 East Main St. Santa Paula, CA 93060, tel. 805-229-7580; e-mail: kbojanowski@sunnybiodiscovery.com;
Abstract
Background: Resveratrol is one of the most popular nutrition supplements, especially in the older population segment, yet its safety and health benefits are not fully established. This study aimed at determining bioavailability and the physiological effects of resveratrol delivered by 2 methods in a one senior patient case study. Methods: Bioavailability of resveratrol and blood chemistry parameters following the gastrointestinal and transbuccal intake were measured, respectively, by HPLC in blood and urine, and by blood chemistry analyzer. Results: The fraction of the initially administered resveratrol detected in the blood was over 15 times higher following transbuccal intake than through the gastrointestinal tract. About 36% of the ingested resveratrol was excreted in urine as 3 major metabolites, while only 11% were secreted as major metabolites following the transbuccal intake. The major metabolite in urine peaked at the same time regardless of the method of delivery. Three long-term (31 day) cycles of resveratrol supplementation by transbuccal mucoadhesive film or by ingestion did not result in any noticeable (over 5%) variation of blood chemistry, blood pressure or the overall physical condition of the patient. Conclusion: Bioavailability of resveratrol delivered through oral mucosa may be over one log higher than by swallowing, as determined by the fraction of the initial resveratrol intake in the blood and, under metabolized form, in urine. Lack of resveratrol-associated changes in blood pressure and chemistry following long term supplementation demonstrates good tolerance to high doses of resveratrol in this senior patient case study.
Key words: Mucoadhesive film, buccal delivery, slow release, biodegradable patch, bioavailability, metabolism, blood, urine.
Introduction
Resveratrol (3,5,4´-trihydroxystilbene) was first isolated by a Japanese scientist Michio Takaoka in 1939 from roots of Veratrum grandiflorum (1). It gained wide attention following the discovery of its cardioprotective (2), chemopreventive (3) and calorie restriction-mimicking potential (4), nearly half a century later.
Similarly to other experimental bioactives, properties of resveratrol have been principally studied in vitro and in animals. Although a database of clinical studies involving resveratrol is growing (5), in depth case studies are rare. Even more uncommon are clinical case studies exploring different methods of administration of resveratrol, although the delivery route and formulation can greatly influence its bioavailability (6). This report addresses bioavailability of resveratrol and its physiological effects following 2 methods of administration – by ingestion (swallowing) and by transbuccal mucosal absorption.
Materials and Methods
Study subject
The study subject was a 74 year old Caucasian male, non-smoker, in generally good health, medicated for hypertension [Ramipril 10 mg and Tialorid® mite ( amiloridi hydrochloridum 2.5 mg + hydrochlorothiazidum 25 mg)], hyperlipidemia [Atorvastatinum 20 mg] , arytmia [Sotaloli hydrochloridum 2×80 mg], mild prostate hyperplasia [Doxazosinum 4 mg and Finaster ( finasteridum 4 mg)] and venous thrombosis [Sintrom (acenocoumarolum) 1-4 mg].
Resverstrol delivery methods
Resveratrol was delivered either by ingestion of the substance powder followed by 100ml water, or by the application of a carboxymethylcellulose-based transbuccal mucoadhesive film containing 34 mg substance through a 9 h continuous delivery period.
Chemicals and reagents
Trans-resveratrol (98% pure) was purchased from Wuhan Yuancheng Technology Development Co., PR China. All other reagents and solvents were analytical grade and obtained, unless otherwise stated, from Sigma-Aldrich (St Louis, MO).
Preparation of the transbuccal delivery system
Resveratrol (1.2g) and Trappsol® (randomly methylated β-cyclodextrin – 3 g) were dissolved in 8 mL methanol, added to a suspension of 6 g carboxymethylcellulose (CMC) in 80 mL of water, mixed thoroughly and poured onto a glass tray to form uniform layer of 580 cm2. After drying at ambient temperature for 3-4 days the resulting mucoadhesive film was cut into 3.5×4 cm strips weighing 0.3 g and containing 34 mg of pure resveratrol, each.
Bioavailability
Delivery of resveratrol for the pharmacokinetic measurement in blood and urine consisted in a single dose of 2g of pure powder swallowed and chased with 100ml of water, or through transbuccal application of 34mg of pure substance formulated in a mucoadhesive film.
Blood was drawn from the median cubital vein and resveratrol content was analyzed as described before (7). Briefly, 2 mL samples were centrifuged to separate plasma from the blood cells. The latter were mechanically disrupted in a Teflon grinder, then both fractions were acidified with 0.1 mL of 6M HCl and extracted with two 4 mL volumes of ethyl acetate. The combined extracts were briefly washed with acidified water and evaporated under vacuum to dryness at room temperature. The residues were dissolved in a combined volume of 0.1 mL methanol, and subjected to HPLC analysis.
Urine samples were collected 1h before resveratrol intake and several times thereafter over a 2 day period. The samples were loaded directly on the column without prior preparation. The time between sample collection and analysis did not exceed 10 hours. Samples were stored at 4°C.
HPLC Measurements
HPLC measurements were performed using an Agilent series 1100 model (Agilent Technologies, Palo Alto, CA) liquid chromatograph equipped with a Rheodyne® 7725 injector, a binary pump, a diode array-UV detection module and a Phenomenex column OOG-4450-EO Luna 250×4.6 mm, 5 µm (Torrance, CA) and ChemStation software. Samples (5µL) were eluted with a 15 min. gradient of 0.05 M H3PO4 (phase B) and pure methanol (phase A) at a flow rate of 1mL/min. Elution was monitored at λ = 308 nm (resveratrol’s peak absorption wavelength).
Method calibration
The HPLC instrument was calibrated with resveratrol (1 to 100 mg/L) in methanol. A linear calibration curve was obtained at the beginning of each series of experiments, with the slope of 0.012-0.015 mg/L per mAU. The extraction recovery was determined on blood samples spiked with known amounts of resveratrol and was found to be 86 ± 5% on the average.
Considering the uncertainties due to incomplete extraction, phase transfer losses and variation of the calibration coefficient, the overall uncertainty of the results are estimated at approx. 30%.
Effects of prolonged delivery
The effects of resveratrol on human body were investigated in 3 one-month cycles. The first cycle consisted in transbuccal absorption of 34 mg of resveratrol from a mucoadhesive film. Transbuccal delivery of resveratrol was obtained by placing a carboxymethylcellulose-based film containing 34 mg of resveratrol on the non-keratinized oral mucosa overnight. The second and third cycles consisted in daily ingestion of resveratrol (180 mg twice daily for cycle 2 and 470 mg once daily for cycle 3). A time interval of 6 months was allowed between the 1st and the 2nd cycle, while the 2nd and 3rd cycle were separated by 6 weeks. Throughout each cycle blood pressure and pulse were measured every morning and blood chemistry tests were performed immediately before and after the cycle. At the same time a physical response to the applied resveratrol supplementation regimen was attempted by self-assessment, which included body weight, physical fitness (walking, running, pushups and pull ups). Some anecdotal attention was also paid to intellectual abilities (crossword puzzles, scrabble and mastermind games).
Data analysis and statistics
Linear trapezoidal method was used to obtain area under the resveratrol concentration-time curve (AUC). The quantification of resveratrol metabolites in urines was based on the resveratrol standard curve. It was assumed that that their recovery characteristics and the ratio of peak areas versus concentrations were the same as for resveratrol and thus they were expressed in resveratrol equivalents, as previously described (14). Two-tailed paired t-test was used for estimating statistical significance of differences between comparisons. Differences were considered significant at p < 0.05 and >15% variation.
Results and Discussion
Buccal intake is the most desirable administration method for many pharmaceutical entities due to the non-invasive, systemic delivery, bypassing gastrointestinal tract and hepatic clearance (8). It may be so as well for resveratrol, which is known to be rapidly and extensively biotransformed in gut and liver (17). Besides enabling metabolic bypass, another advantage of transmucosal administration is locoregional support of tissues, such as hypodermis (9, 10). Therefore, we set to compare transbuccal and gastrointestinal administration of dietary – relevant doses (13) of resveratrol in a clinical case study with a senior patient. For the transbuccal delivery, among several methods of enhancing resveratrol solubilization, such as micelles, micronization or cyclodextrins, beta cyclodextrin was selected (16). Transfer of free resveratrol to the bloodstream following intestinal and transbuccal intake routes was compared by measuring its concentration in the whole blood samples at different time points. Blood sample processing as described above enabled the detection of the absorbed resveratrol through standard HPLC method [Fig.1, compare 1B to resveratrol standard (1A)]. The dose delivered by the mucoadhesive film (34 mg) represented only 1.7 % of the amount administered orally (2 g), hence much lower signal recorded (compare Fig. 1B with 1C).
Blood pharmacokinetics of ingested resveratrol are presented in Table I. They are in agreement with previous reports of peak concentrations (Cmax) of about 0.3 μg/mL detected at time of maximal concentration (Tmax) of 0.8-1.5h post dose with AUC of ~800 ng*h/ml (for a dose of 2.5mg) (14, 15). Ingested resveratrol appeared in the blood faster than the transbucally absorbed material. This is possibly due to the slow release kinetics of this resveratrol-loaded mucoadhesive film. However, after 2h, the amount of transbuccal resveratrol in the bloodstream surpassed the gastrointestinal route in terms of the % of the administered dose by fifteen-fold, reaching 0.61% vs. 0.04% for the ingested resveratrol. Taken together, these results suggest that more bioavailable resveratrol may proportionally be absorbed through oral mucosa compared with ingestion.
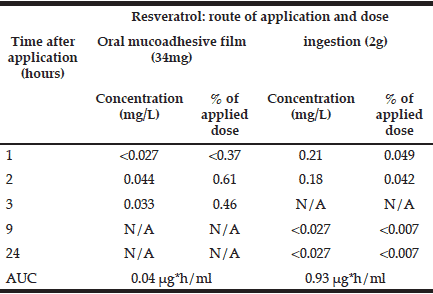
Table 1 Time-course of resveratrol in blood after transbuccal (34mg) and gastro-intestinal (2g) intake, assuming total blood volume of 4.7 L
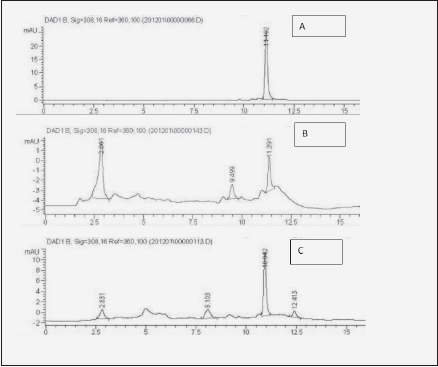
Figure 1 Illustration of blood HPLC chromatograms used for Table I. A: Resveratrol standard (10 µg/mL); B: blood 2h after transbuccal administration of resveratrol through mucoadhesive carboxymethylcellulose-based film; C: blood 2 h after ingestion of 2 g of resveratrol. The peak eluting at 2.8 min. is inherent to prepared blood (7)
Figure 2 illustrates the typical HPLC readout of urinary excretion of ingested resveratrol and its metabolites. Fig. 2A shows the elution profile of pure resveratrol standard (20 mg/L Tret=11.2 ± 0.2 min) and Fig. 2B – of urine before resveratrol ingestion. Fig. 2C shows the elution profile of urine spiked with the resveratrol standard. Again, a distinct peak of resveratrol appears at Tret= 11.1 min., indicating lack of interference from other urine components with resveratrol retention. Fig. 2D is the elution profile of urine collected 8.5 hours following ingestion of 2 g resveratrol powder. In this spectrum, in addition to the small peak of resveratrol (Tret = 11.2 min.), three large peaks [Tret = 8.8 min., 9.5 min. and 12.3 min (+/-0.2 min)] can be seen, which are absent from a normal urine. They are likely resveratrol metabolites and although their chemical identity has not been established here, previous studies indicate that they belong to the family of more polar glucuronide followed by less polar sulfate conjugates (6-9, 14, 15).
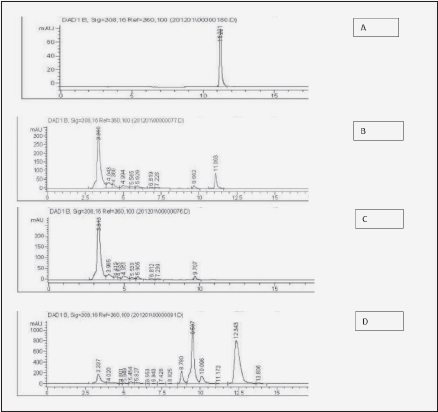
Figure 2 Illustration of urine HPLC chromatograms used for Figure 3. A: pure resveratrol standard (20 μg/ml); B: urine spiked with resveratrol standard (20 µg/mL); C: urine before ingestion of 2 g of resveratrol; D: urine 8.5 h after ingestion of 2 g of resveratrol showing glucuronide (less polar) followed by sulfate (more polar) conjugates, with the peak eluted at 12.3 min being the most prominent metabolite - resveratrol-3-O-sulfate, according to previous reports (13-15)
Figure 3 shows that the time course of urinary metabolite excretion is similar regardless of the absorption route, peaking at about 6-8 h. This suggests that the delivery of resveratrol through this particular transbuccal film resulted in similar time of systemic exposure to the substance as through intestinal absorption. Interestingly, the ratio of Metabolite 3 vs. 2 seems to be higher following transbuccal delivery, although this may be an artifact due to low signal. Compared with the ingestion route, smaller proportion of transbucally-absorbed resveratrol was detected as metabolites in the urine [ingestion of 2g of resveratrol resulted in a total metabolite excretion of 711 mg (35.5% of the ingested resveratrol, Fig. 3A), while transbuccal delivery yielded only 3.6 mg of metabolites in the urine (10.6% of the transbucally delivered resveratrol) (Fig. 3B)] This occurred despite higher % of the transbucally-administered compound detected in the bloodstream (Table I). This may signify that comparably more of the transbucally absorbed resveratrol was utilized by tissues in its native form instead of being metabolized, which would indicate better bioavailability of the transbuccal resveratrol. Also, interestingly, the finding that the bioavailability of transbucally-delivered resveratrol is ~15 times better than through gastrointestinal absorption substantiates the custom of holding and turning wine in the mouth before swallowing it, common among wine connoisseurs.
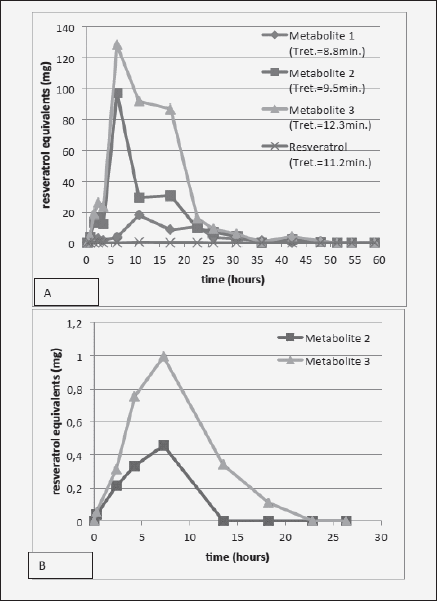
Figure 3 Time-course of urinary excretion of metabolites following either (A) 2 g resveratrol ingestion (time 0); or (B) transbuccal absorption from a mucoadhesive film formulated with 34 mg of resveratrol and applied to the non-keratinized oral mucosa (time 0)
The safety and clinical benefit of resveratrol supplementation is still not settled (11, 12). Therefore, every new clinical case report is valuable, especially from the more fragile, senior population, which may use more resveratrol supplementation than other age groups. Following the comparative study between the 2 routes of absorption, the study subject embarked on a long-range dose-response study divided in 3 cycles. Cycle 1 consisted in a daily intake of 34 mg of resveratrol through the mucoadhesive film, overnight for the duration of 1 month. This was followed by 6 months of respite and 2 one-month cycles of resveratrol ingestion (0.36 and 0.47 g/day respectively) separated by a 6 weeks interval (Table 2). It was found that none of the measured parameters consistently varied by > 5% (p value<0.05). Notably, parameters commonly monitored in seniors, such as weight, blood pressure, blood glucose, cholesterol, high density lipoprotein and triglycerides remained unchanged, demonstrating a robust tolerance to high doses of resveratrol. Considering the human data consistent with chemopreventive effects of resveratrol and/or its metabolites (15), our results indicate that chemoprevention-oriented high-dose resveratrol supplementation may be safe for seniors.
Table 2 Effects of long term supplementation with resveratrol on blood chemistry and blood pressure. Resveratrol was administered in three one-month cycles separated by 6 months (between Cycle 1 and 2) and 6 weeks (between cycle 2 and 3). P value was > 0.05 for all blood parameters tested
Acknowledgements: We would like to thank Stephanie Ma for her outstanding technical assistance and Dr. Zbigniew Radecki for helpful discussions.
Conflict of Interest: Authors state no conflict of interest.
Ethics Statement: This clinical case study complied with the international ethical guidelines for biomedical research involving human subjects established by the Council for International Organizations of Medical Sciences (CIOMS).
References
1. Takaoka M. Resveratrol, a new phenolic compound from Veratrum grandiflorum. J Chem Soc 1939;60:1090–1100.
2. Renaud S, M de Lorgeril. Wine, alcohol, platelets and the French paradox for coronary heart disease. The Lancet 1992;339:1523–26.
3. Jang M, Cai L, Udeani GO, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997;275:218–20.
4. Chung JH, Manganiello V, Dyck JR. Resveratrol as a calorie restriction mimetic: therapeutic implications. Trends Cell Biol 2012;22:546.
5. Clinicaltrials.gov
6. Smoliga JM, Blanchard O. Enhancing the delivery of resveratrol in humans: if low bioavailability is the problem, what is the solution? Molecules 2014;19:17154-72. doi:10.3390/molecules191117154.
7. Biasutto L, Marotta E, Garbisa S, Zoratti M, Paradisi C. Determination of quercetin and resveratrol in whole blood–implications for bioavailability studies. Molecules 2010;15:6570-9. doi: 10.3390/molecules15096570.
8. Giannola LI, Sutera FM, De Caro V. Physical methods to promote drug delivery on mucosal tissues of the oral cavity. Expert Opin Drug Deliv 2013;10:1449-62.
9. Bojanowski K. Hypodermal delivery of cosmetic actives for improved facial skin morphology and functionality. Int J Cosmet Sci 2013;35:562-7.
10. Zhao H, Bojanowski K. Hypodermis: the last frontier for facial cosmetics. H&PC Today 2014;9:38-42.
11. Novelle MG, Wahl D, Dieguez C, Bernier M, de Cabo R. Resveratrol supplementation: Where are we now and where should we go? Ageing Res Rev 2015;S1568-1637(15)00004-5.
12. Park EJ, Pezzuto JM. The pharmacology of resveratrol in animals and humans. Biochim Biophys Acta 2015;S0925-4439(15)00023-X. doi: 10.1016/j.bbadis.2015.01.014.
13. Walle T. Bioavailability of resveratrol. Ann NY Acad Sci 2011;1252:9-15.
14. Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher AJ, Steward WP, Brenner DE. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007;16:1246-52.
15. Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, Vasilinin G, Sen A, Schinas AM, Piccirilli G, Brown K, Steward WP, Gescher AJ, Brenner DE. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010 Nov 15;70:9003-11. doi: 10.1158/0008-5472.CAN-10-2364.
16. Ansari KA, Vavia PR, Trotta F, Cavalli R. Cyclodextrin-based nanosponges for delivery of resveratrol: in vitro characterisation, stability, cytotoxicity and permeation study. AAPS PharmSciTech. 2011;12:279-86. doi: 10.1208/s12249-011-9584-3.
17. Wenzel E, Somoza V. Metabolism and bioavailability of trans-resveratrol. Mol Nutr Food Res. 2005 May;49:472-81.
